Reprint: The core data of this paper - national minimally invasive surgical instrument policy, provincial and municipal minimally invasive surgical instrument policy, policy interpretation
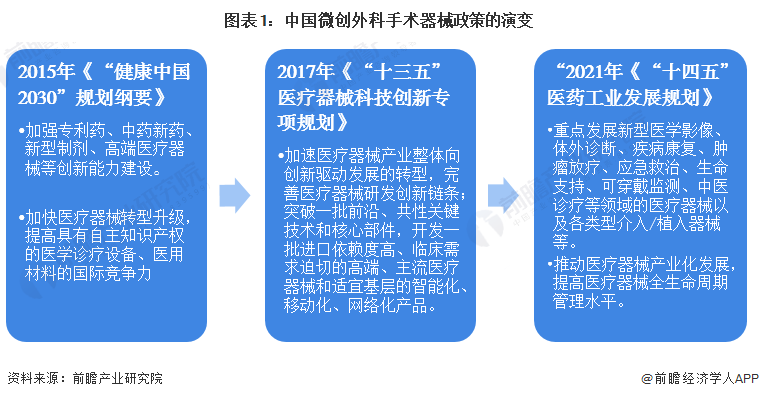
1. China's minimally invasive surgical instrument industry policy history chart
From the perspective of policy changes related to minimally invasive surgical instruments, the policy focus around 2015 is on improving the independent research and development capacity of medical devices and the construction of innovation capacity. During the "13th Five-Year Plan" period, it began to pay attention to the research and development chain of medical devices, and proposed to speed up the process of localization of medical device products, and develop intelligent, mobile and networked products. During the "14th Five-Year Plan" period, the key development areas were identified, including new medical imaging, in vitro diagnosis, disease rehabilitation, tumor radiotherapy, emergency treatment, life support, wearable monitoring, traditional Chinese medicine diagnosis and treatment. Start to pay attention to the whole life cycle management of medical devices.
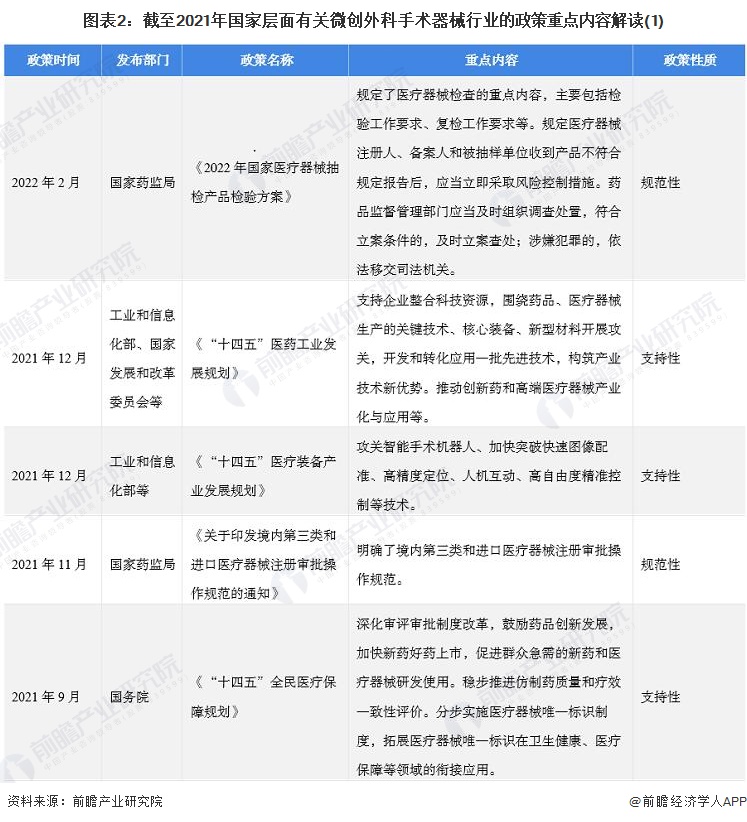
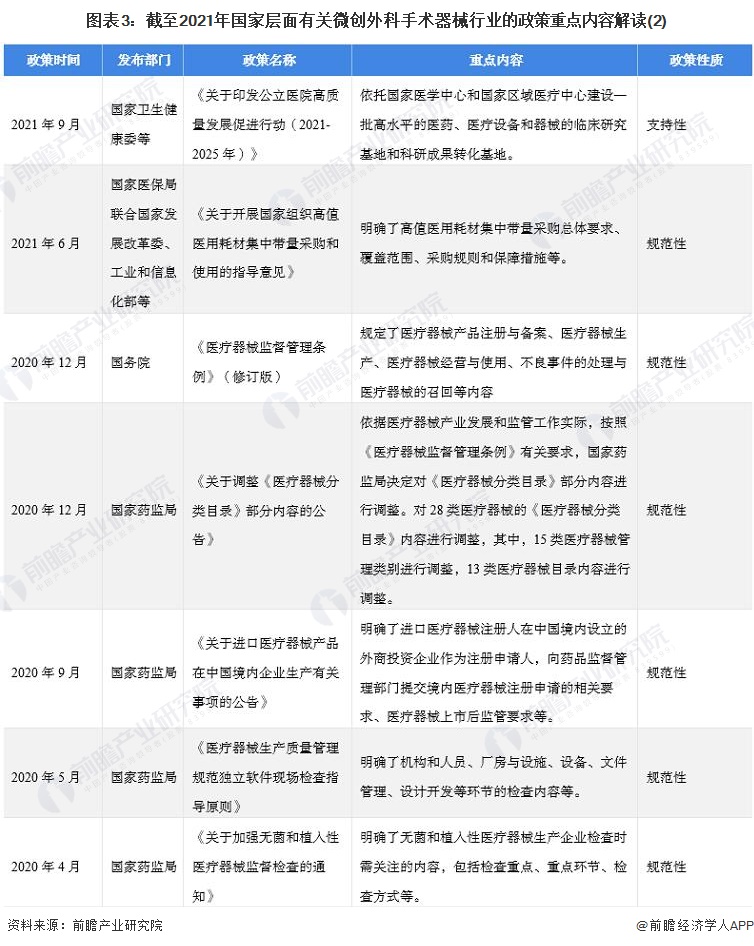
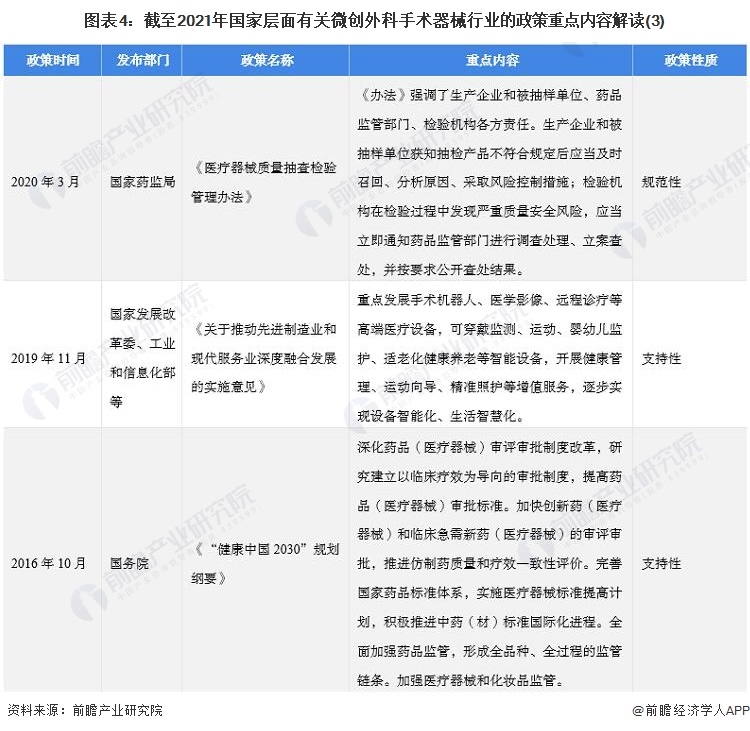
2. Summary and interpretation of minimally invasive surgical instrument industry policies at the national level in China
-- Summary of minimally invasive surgical instrument industry policies at the national level in China
At present, the policies related to minimally invasive surgical devices at the national level are mainly normative documents, including the "National Medical Device sampling Product Inspection Program in 2022", "Notice on the issuance of Domestic Class III and Imported Medical Device Registration and Approval Procedures", and some supporting documents. Mainly for the "14th Five-Year" pharmaceutical industry Development Plan ", "14th Five-Year" medical equipment industry Development Plan "and so on.
2) Interpretation of the "14th Five-Year Plan" for the Development of Pharmaceutical Industry
On December 22, 2021, the Ministry of Industry and Information Technology, the National Development and Reform Commission and other departments jointly issued the "14th Five-Year Plan for the Development of the pharmaceutical Industry", which clearly proposed the development of high-end medical devices and improve the quality management level of the full life cycle of medical devices and product quality.
The main contents of the "14th Five-Year Plan" Pharmaceutical Industry Development Plan are as follows:

3) Interpretation of the "14th Five-Year Plan" for the Development of Medical Equipment Industry
On December 21, 2021, the Ministry of Industry and Information Technology, the National Health Commission and other departments jointly issued the "14th Five-Year Plan" Medical equipment Industry Development Plan, which clearly proposed the development goals during the "14th Five-Year Plan" period: By 2025, the foundation of the medical equipment industry will be developed to a higher level, the modernization of the industrial chain will be enhanced, the supply capacity of mainstream medical equipment will be enhanced, and the performance and quality of high-end medical equipment products will be improved. In the field of minimally invasive surgical instruments, it is proposed to promote the development of precision, minimally invasive, rapid, intelligent and reusable treatment equipment.

4) Summary of relevant policies in China's surgical robot industry
In recent years, surgical robots have begun to enter the public eye. Surgical robot is a kind of precision medical equipment, which is invented with the help of the development of micro-trauma surgery and related basic technology, and has more accurate operation ability in minimally invasive surgery, so it is developing rapidly in many countries. China attaches great importance to the development of surgical robots, and the state has successively issued some policies to promote the research and development and manufacturing of surgical robots. The main policies are summarized as follows:

3. Summary and interpretation of minimally invasive surgical instrument industry policies at the provincial and municipal level in China
-- Summary of minimally invasive surgical instrument industry policies in 31 provinces and cities in China
At present, a number of provinces and cities, including Jiangsu, Zhejiang, Shandong, Guangdong, Shanghai, Beijing, etc., in the "14th Five-Year Plan" period issued minimally invasive surgical instruments related policies, clear development goals and other content, the main content is as follows:
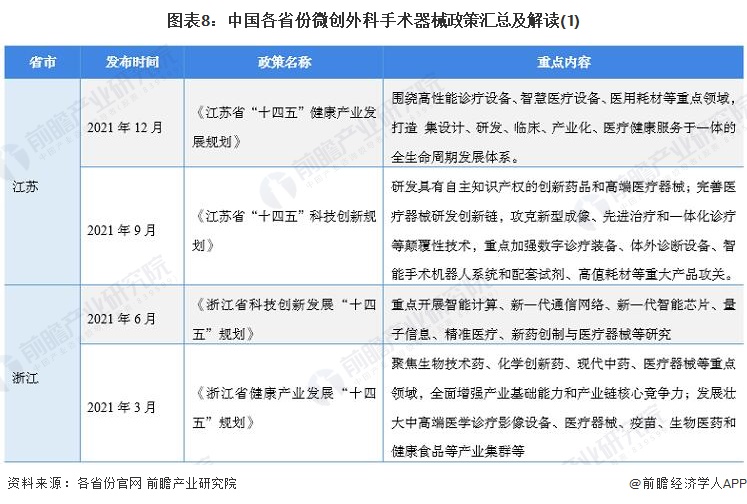
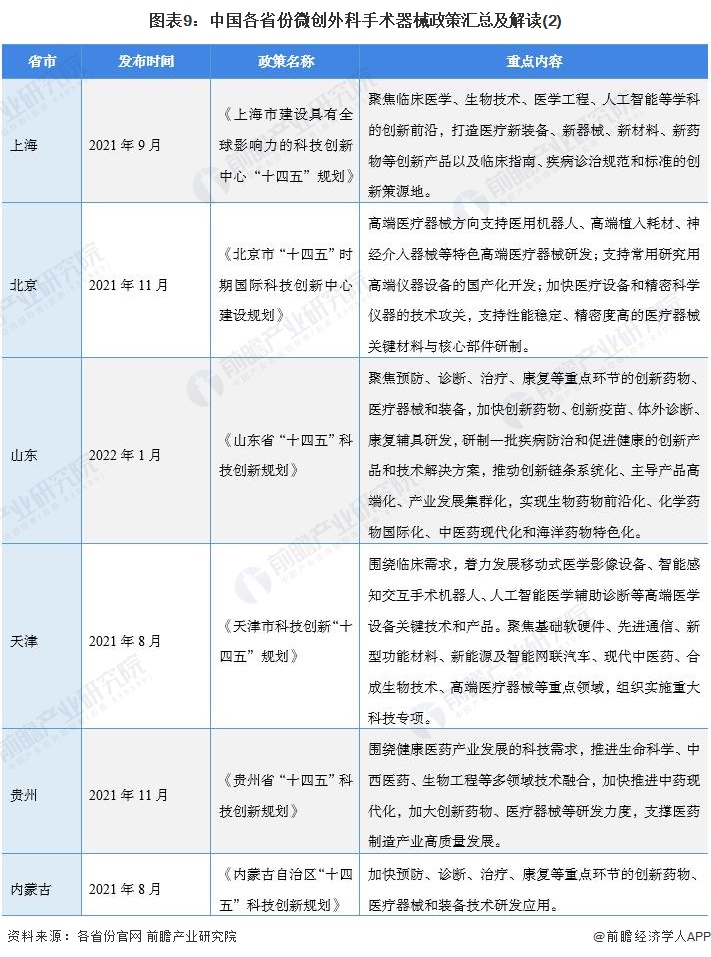
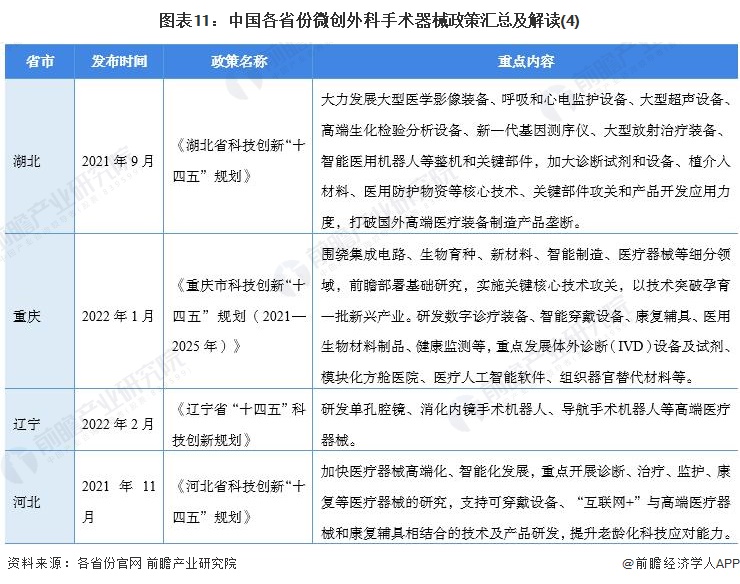
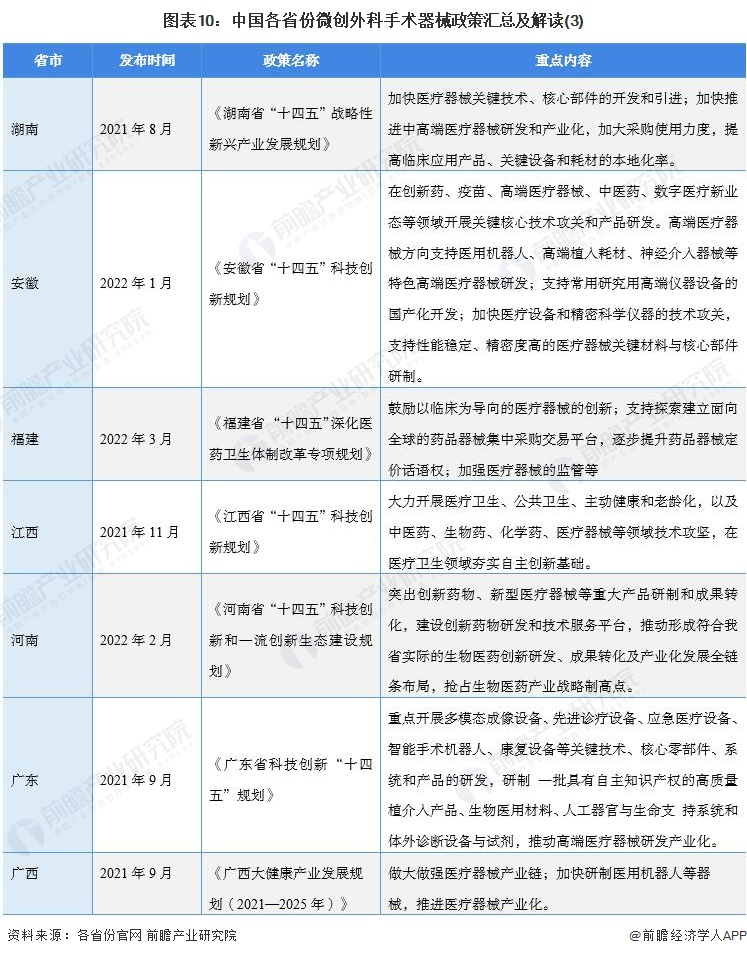
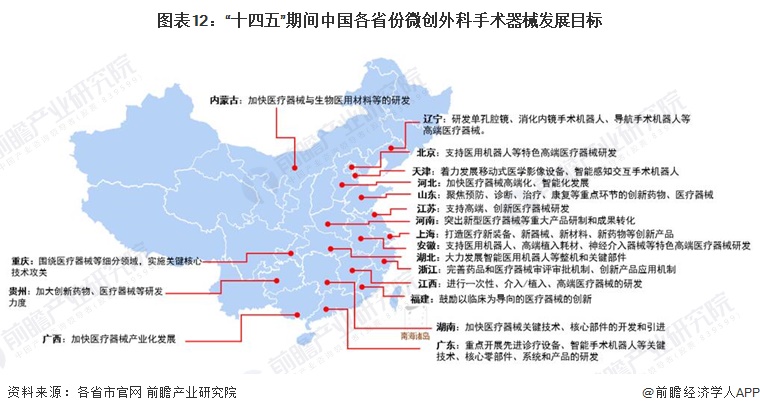
2) Interpretation of the development goals of minimally invasive surgical instrument industry in 31 provinces and cities in China
During the "14th Five-Year Plan" period, China's major provinces also put forward the development goals of the minimally invasive surgical instrument industry, including: supporting the research and development of high-end medical devices, developing surgical robots, and carrying out core technology research. The details are as follows:
The above data and analysis are referred to the "China Minimally Invasive Surgical Instrument Industry Market Outlook and Investment Strategic Planning Analysis Report" of Prospective Industry Research Institute. At the same time, the Prospective Industry Research Institute also provides industrial big data, industrial research, policy research, industry chain consulting, industry map, industrial planning, park planning, industrial investment promotion, IPO fundraising research, IPO business and technology writing, IPO working paper consulting and other solutions.
|
Last:Reprint: Market segment analysis of China ophthalmic high-value consumables industry in 2024
Next:Appearance and function examination of surgical instruments |
Return |