Source: Photo network
Inspired by leeches, researchers in Switzerland released a new blood-collecting device a few months ago. The device does away with needles, which many fear, and instead uses a combination of suction cups and microneedles to get enough blood with as little trauma as possible.
Leeches always thought they had the attention of the scientific community. Leeches release an enzyme called hirudin, which is a powerful anticoagulant. When the leeches fall off after eating, hirudin will still play a role in the bite site, and the wound of the host will continue to bleed for some time. And after the leech has eaten enough to leave, the host's blood in its digestive tract can remain unclotted for a long time. Researchers have previously found that some leeches can live for a year on a single suck of blood!
Now, blood collection devices modeled on leeches are expected to help people collect blood more easily. Bloodsucking leeches have a suction cup in their mouth and many sharp "small teeth" in their mouth, and suck blood by swallowing to create negative pressure when eating. In this context, in a study published by ETH Zurich in March this year, the suction cups and microneedles of the new blood collection device mimic the mouth suction cups and small teeth of leeches. The sucker is attached to the patient's arm, and when pressed, the microneedles inside the sucker will puncture the skin. After that, the release of pressure in the sucker creates negative pressure, which draws the blood in and holds it in a storage space containing anticoagulants.
Microneedles are less invasive, cause less pain and discomfort, and wounds heal more easily. In addition, because the microneedles are fixed inside the suction cup, compared with traditional blood collection needles, this reduces the risk of injury during the puncture process and subsequent treatment. The researchers are also developing a new version of the device made from fully biodegradable materials.
Non-medical professionals can also use this blood collection device. And compared to fingertip blood collection, it can collect more blood, improving the accuracy of diagnosis. In addition, it can be manufactured at a low cost and is easy to carry, so it is suitable for low - and middle-income areas, and it is expected to help people better fight diseases such as malaria.
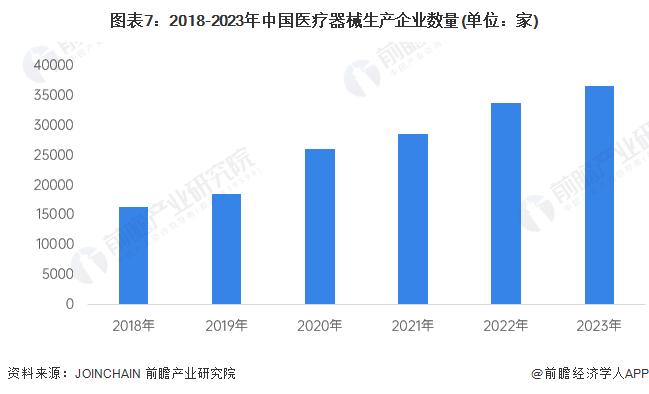
There are more than 36,000 medical device manufacturers
In recent years, the number of medical device manufacturers in China has shown a rapid upward trend. By the end of 2023, there were more than 36,000 medical device manufacturers in China, with a compound growth rate of more than 17% from 2018 to 2023.
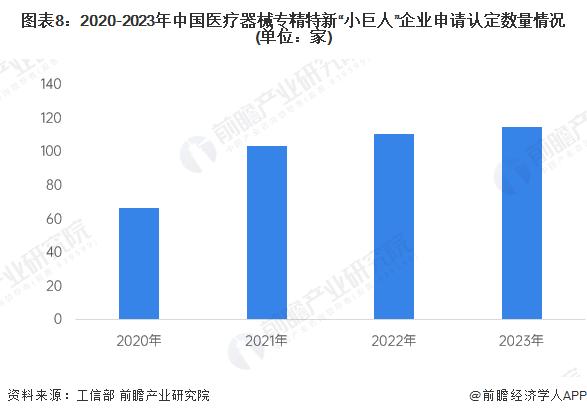
Since the Ministry of Industry and Information Technology carried out the identification of the first batch of specialized and special new "small giant" enterprises in 2019, the number of medical device specialized and special new "small giant" enterprises in China has maintained stable growth. In 2023, a total of 115 medical device companies in the country successfully identified specialized special new "little giant". By the end of 2023, China has a total of 397 specialized and new "little giant" enterprises based on medical devices.
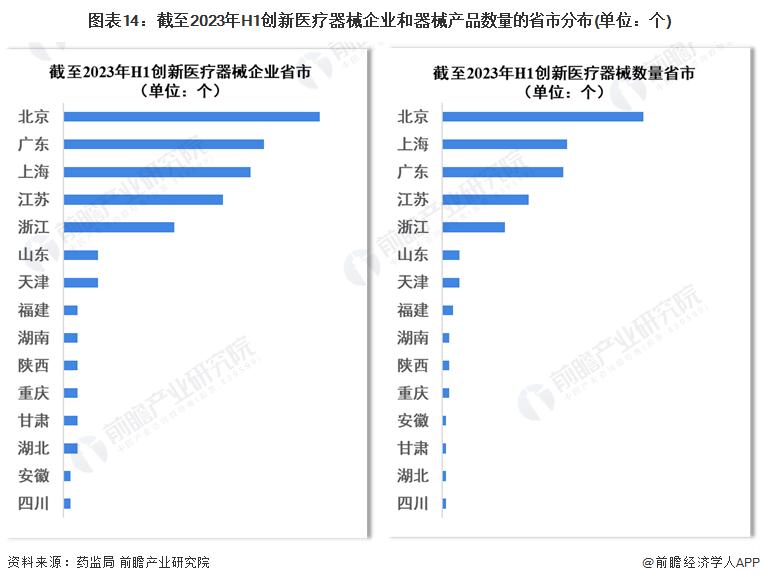
Regional distribution of China's innovative medical device innovation approval
In the existing 217 innovative medical device approval projects, Beijing, Shanghai, Guangdong, Jiangsu, Zhejiang innovative medical device approved products and the number of corresponding enterprises are the largest.
Li Yingjun, general manager of Beijing Tonghe Litai Biotechnology Co., LTD., said in his speech "The General Idea of Innovative Research on Medical Devices" that "the core of innovative medical devices lies in the technological breakthrough and the practical value of clinical application." He stressed that the risk of innovative medical devices is gradually reduced during the research and development process, and the research and development process is rigorous and meticulous. "From concept to commercialization, scientific processes and standards must be strictly followed at every step to ensure the safety and effectiveness of the product," he noted. Li Yingjun said: "With the progress of technology and policy support, innovative medical devices will usher in greater development opportunities in the future. We should seize the opportunity and continue to innovate to make greater contributions to the cause of human health."
|
Last:Reprint: Overseas Medical Device Product Registration Report for the first half of 2024 (below)
Next:Reprint: Medical Equipment Procurement Analysis Report for the first half of 2024 |
Return |