Core data: Medical accelerator; Industrial chain; Investment trend; Regional distribution
Overview of the medical linear accelerator industry chain: Breaking high technical barriers is the key
The industrial chain of China's medical linear accelerator industry can be divided into three links, the upstream participants of the industrial chain are raw materials and parts suppliers, which can be divided into core components, auxiliary operation software and other parts suppliers; The middle reaches of the industrial chain are medical linear accelerator manufacturers, providing product design and development, manufacturing, sales and maintenance services; The downstream of the industrial chain is the application site of the medical linear accelerator and the terminal patient.
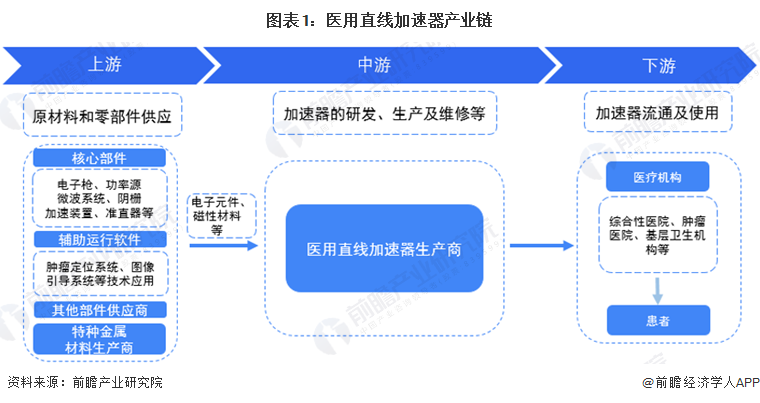
Upstream manufacturers include medical equipment raw materials, electronic devices, mechanical manufacturing and other components/materials manufacturers, of which the biggest impact on the medical linear accelerator industry is the electron gun, microwave system, acceleration tube and other core components manufacturers. The representative companies include Meda, Varian, Siemens, STAIB Instruments, Kimball Physics, Electrovision, Gotron Equipment, etc. At present, the international development and production of medical linear accelerator companies are mainly medical Da, Varian, Zhongke super fine, Xinhua Medical, Lianying Medical, etc. In recent years, the cumulative number of medical electronic linear accelerator registrations in China has risen steadily, but in general, the current Chinese medical linear accelerator industry midstream manufacturers are mainly foreign companies. The downstream market is broad, including various types of medical institutions such as hospitals and health institutions at all levels, as well as many cancer patients.

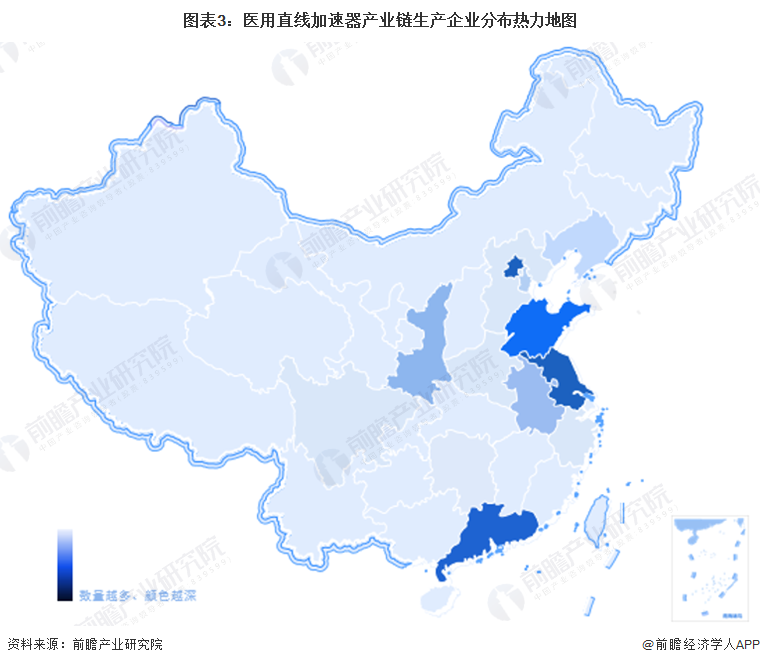
Regional thermal map of medical linear accelerator industry chain: Jiangsu has the most concentrated distribution
From the regional distribution of China's medical linear accelerator industry chain enterprises, medical linear accelerator industry chain enterprises are mainly distributed in Jiangsu, followed by Shaanxi, Shandong, Beijing, Guangdong and other regions; In other places, such as Fujian, Gansu, Jilin, Jiangxi and other provinces, although there are enterprises distribution, but the number is small.
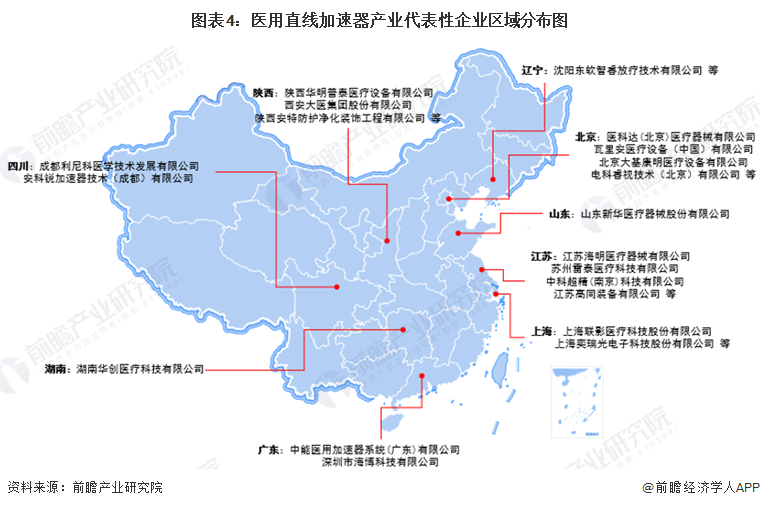
From the perspective of the distribution of representative enterprises, there are more representative enterprises in Beijing, Guangdong, Shanghai, Jiangsu and other places, while Shaanxi also has more representative enterprises, such as Shaanxi Huaming Putai Medical Equipment Co., LTD., Xi 'an Medical Group Co., LTD.
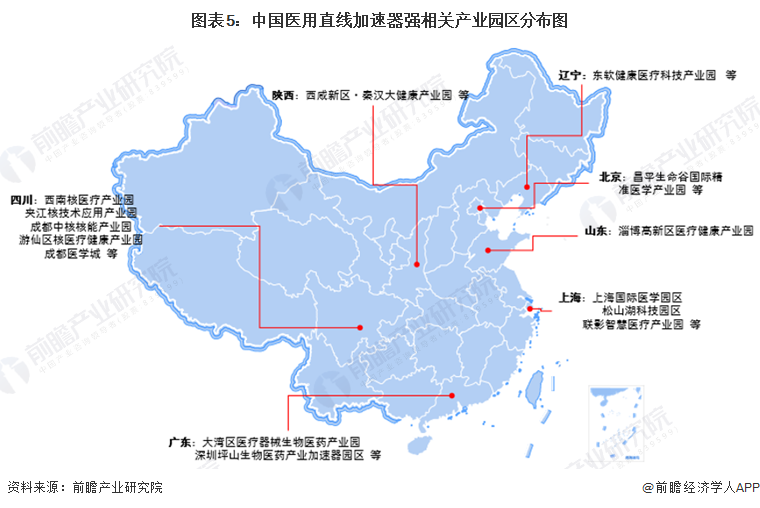
Medical linear accelerator industrial park distribution: relying on nuclear technology application industry layout
Because the medical linear accelerator industry is a very small classification of subdivisions of the industry category, at present, most of its industry-related enterprises or manufacturers rely on high-tech industrial development park, medical device industrial park or medical health industrial park and other industrial park development and construction, in addition, medical linear accelerator electron gun, accelerator tube and other core components and nuclear technology applications are closely related. Therefore, the following statistics are based on the situation of medical linear accelerator manufacturers or the main cooperative parks of nuclear technology institutes.
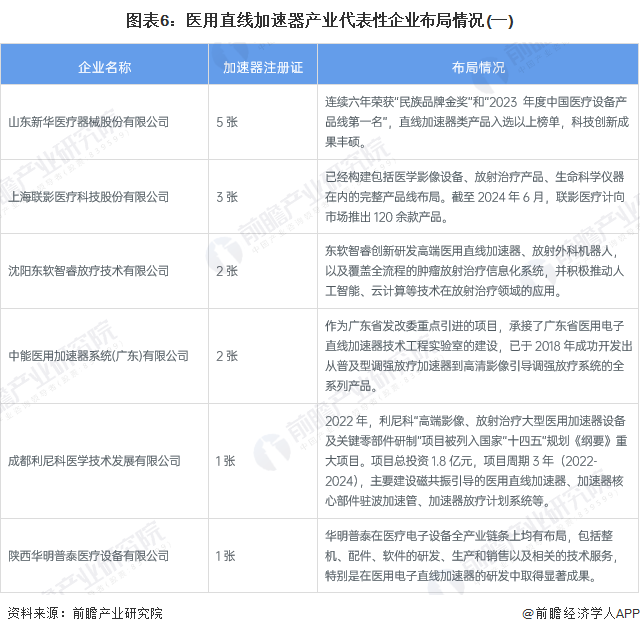
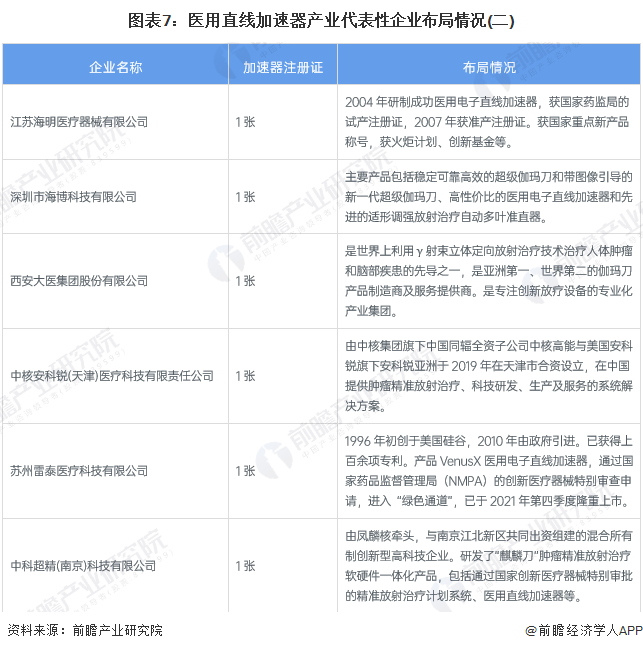
Medical linear accelerator representative enterprise layout
At present, the layout of the medical linear accelerator manufacturing business of China's listed enterprises, mainly Shandong Xinhua Medical Equipment Co., Ltd. and Shanghai Lianying Medical Technology Co., LTD., in addition, There are also more than 10 unlisted companies such as Shenyang Neusoft Zhiru Radiotherapy Technology Co., LTD., Zhongneng Medical Accelerator System (Guangdong) Co., LTD., which hold medical linear accelerator registration certificates and can produce effective medical linear accelerator products. The layout of representative enterprises in the medical linear accelerator industry is as follows:
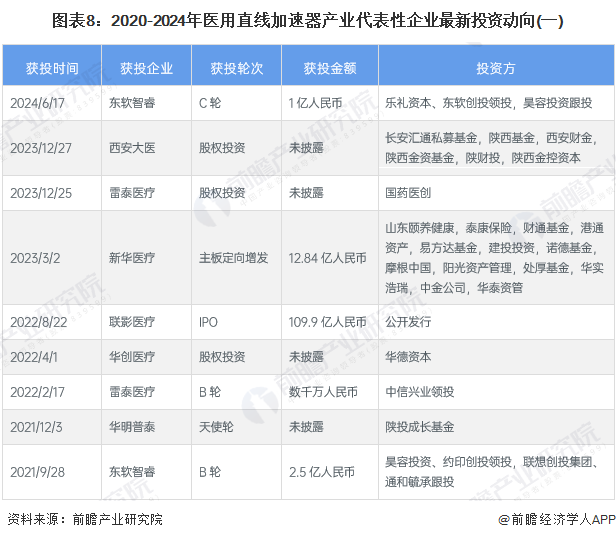
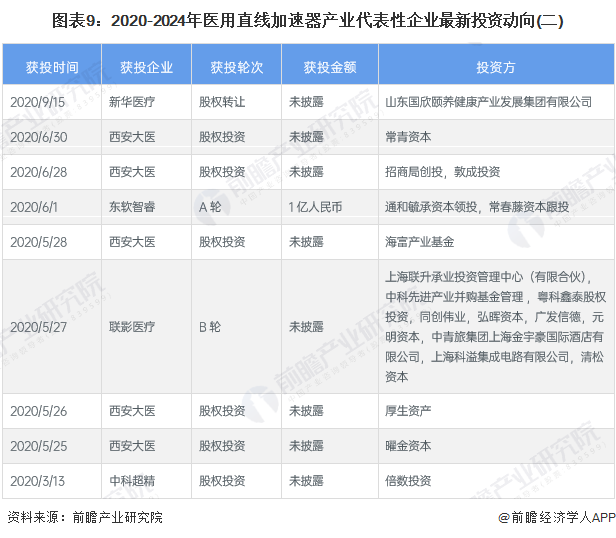
The latest investment trend of representative enterprises in medical linear accelerator industry
Since 2020, the investment trends of representative enterprises in China's medical linear accelerator industry mainly include the acquisition of companies to expand business and investment projects through capital increase of subsidiaries. In recent years, there have been many investment and financing events in the industry, and large enterprises and start-ups have shown a rapid development momentum. The latest investment trends of representative enterprises in the medical linear accelerator industry are as follows:
|
Last:Reproduced:IBoston Scientific "2 Medical Devices" approved by FD
Next:reship:Ciie: The big "show" of global medical device New products |
Return |